Announcement posted by Geneseq Biosciences 06 Feb 2019
5 February 2018
-- New research, published today, in PLOS ONE shows that the Melaseq™ test can also be used on skin biopsy tissue.
-- Retrospective analysis of patient data suggests that Melaseq may be able to identify good/poor prognosis patients at the time of diagnosis - allowing doctors to individualise treatment plans.
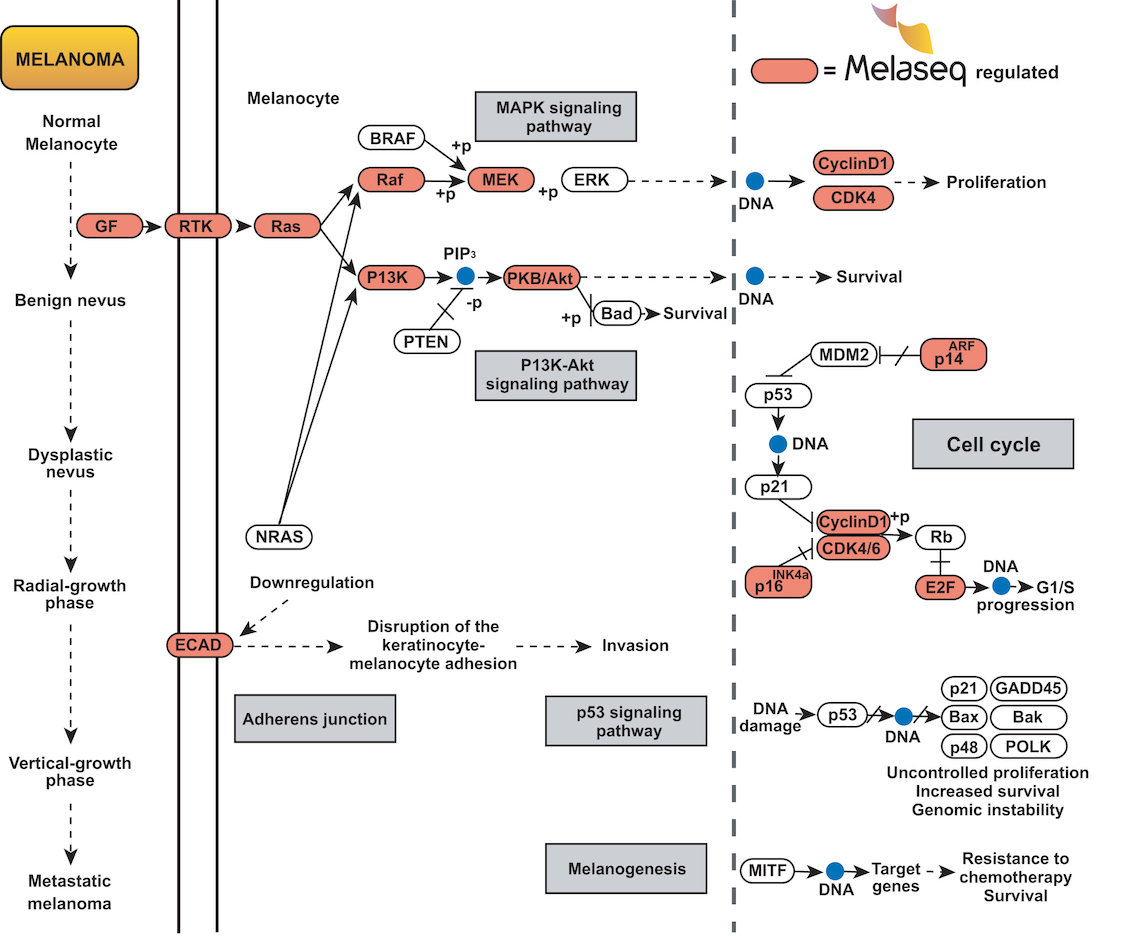
-- Investigation into the biological role of the 38 Melaseq microRNAs revealed significant regulation of genes cruicial to melanoma development and metastasis.
Research identifying a new way to get more accurate results from skin cancer biopsies has recently been published by the Public Library of Science (PLOS ONE).
The new test, Melaseq, liquifies tissue taken from a suspected melanoma and runs it through a genetic test created by Melbourne start-up company, Geneseq.
Currently, melanoma biopsies are diagnosed through visual assessment of microscopic slides by pathologists, resulting in up to 15% uncertain or incorrect diagnoses.
The Melaseq test is a more objective testing method that relies on uniform data and information for diagnosis. This will improve melanoma diagnostic accuracy, where uncertainty exists due to the challenging nature of skin lesion pathology.
This means more potential for earlier diagnosis and less room for error.
The research also showed that Melaseq may also predict a patient’s length of survival. Patients with a genetically ‘high risk’ Melaseq result have a more aggressive form of melanoma may choose to begin treatment earlier, potentially preventing the cancer from spreading.
Geneseq Director and creator of the test Dr Ryan Van Laar said that Melaseq had the potential to be the new ‘gold standard’ of melanoma diagnosis.
“By using established science and existing data we can provide a more accurate test result that doctors and patients can rely on, rather than the subjective visual assessment by an individual pathologist.”
“The difficulty of accurately diagnosing melanoma from visual assessment only is demonstrated in the fact that up to 15% of samples sent to pathology for assessment come back without a diagnosis at all,” said Dr Van Laar.
According to the research paper, the worldwide incidence of melanoma skin cancer has continued to increase at a rapid rate over the last 30 years.
In 2015, approximately 351,880 melanoma skin cancers were diagnosed globally.
This is the second application of the skin cancer diagnostic test developed by Geneseq, first published in the British Journal of Cancer in 2018.
The Melaseq test can identify disease-specific microRNA expression, in either biopsy tissue or blood. This assists in the diagnosis and prognosis of melanoma more accurately, and at a much earlier stage, than previously possible.
“To our knowledge, this is the first time that this method of testing has been applied to genomic profiles of both blood and solid tissue,” said Dr Van Laar.
“While a blood test may be the first option for doctors in diagnosing melanoma, Melaseq-FFPE will help pathologists looking at tissue samples that have already been sent it in for assessment.”
“We look forward to this research becoming clinically available and helping healthcare professionals identify the disease in its earliest and most curable stage,” Dr Van Laar said.
About Melanoma
Two in three Australians will be diagnosed with skin cancer during their lifetime, of which melanoma is the rarest but most deadly form. Worldwide, the incidence of melanoma continues to increase, outpacing the rise of any other malignancy in the Caucasian population over the last 30 years.
Despite being curable if diagnosed early, four Australians die each day from this cancer, resulting in more deaths than the road toll.
If not diagnosed early and accurately, melanoma grows quickly spreads through the lymphatic system and bloodstream to other organs such as the lungs, liver, brain or bone. There are currently no specific solid tissue, blood or genetic tests available to help doctors diagnose melanoma or monitor for recurrence.
In 2018 there will be an estimated 14,320 melanoma diagnoses, 1,905 deaths and over 50,00 people living with the disease. Over 2 million Australians have one or more high-risk characteristics for developing melanoma in their lifetime.
About Geneseq Biosciences:
Geneseq Biosciences is a Melbourne based biotechnology company, pioneering the development of new “liquid biopsy” tests to help doctors individualise cancer diagnostics & treatment options.
Founded in 2016, their first test Melaseq, is designed to accurately detect the genetic (microRNA) fingerprint of active melanoma in blood.
Geneseq Biosciences was selected to participate the 2017 Melbourne Health Incubator program and published their first study in the British Journal of Cancer in January, 2018.
For more information please contact Dr Ryan van Laar, Geneseq Biosciences, +61 (3) 9028 2992
Email: info@geneseq.com.au| Web: www.geneseq.com.au | Twitter: @geneseqbio
Publication link: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0211504

